Mg(s) +  O2(g) → MgO(s) + 601.6 kJ ΔH = −601.6 kJ/mol
O2(g) → MgO(s) + 601.6 kJ ΔH = −601.6 kJ/mol
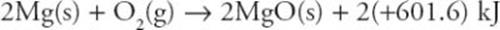
莫爾數就是係數嗎?
第一式 coefficient times 2 =第二式 兩個方程式還是一樣的嗎?
為甚麼kj焦耳也要跟著乘二?
請提共詳細解釋
ㄑcombination reaction 有一定就是放熱還是吸熱反應? 為甚麼combination reaction 的 heat of formation is negative???
decomposition reaction 有一定就是放熱還是吸熱反應? 為甚麼decomposition reaction 的 heat of formation is also negative???
If the heat of formation is a high exothermic (ΔH is negative) value, the compound will be difficult to decompose since this same quantity of energy must be returned to the compound. A low heat of formation indicates decomposition would not be difficult???
為甚麼translate and explain
如果形成熱是高放熱,則化合物將難以分解,因為該相同量的能量必須返回到化合物。 低的生成熱分解不困難 甚麼意思
